Introduction
The term “Nylon 6” is more than just a name — it holds critical information about the chemical structure, raw materials, and properties of the polymer. This numeric designation plays a central role in the classification and differentiation of various types of nylon, a family of synthetic polyamides that revolutionized materials science in the 20th century.
In this article, we’ll unpack what the “6” in Nylon 6 means from a chemical, industrial, and historical standpoint and explore how it compares with other nylons, such as Nylon 6,6. By the end, you’ll understand how a single number can offer deep insight into the building blocks of modern polymer chemistry.
1. What Is Nylon? A Brief Overview
Nylon is a synthetic thermoplastic polymer, specifically a polyamide, which means its molecular chains are held together by amide bonds (-CONH-). First synthesized in the 1930s by Wallace Carothers at DuPont, nylon was initially intended as a synthetic alternative to silk and quickly found use in textiles, military applications, and later in engineering plastics.
There are many types of nylon, including:
Nylon 6
Nylon 6,6
Nylon 6,10
Nylon 11
Nylon 12
Each variant has a different molecular structure, resulting in distinct properties and uses.
2. Decoding the “6” in Nylon 6
2.1 Source Monomer: Caprolactam
The “6” in Nylon 6 refers to the number of carbon atoms in the monomer from which it is synthesized. Nylon 6 is made from a single monomer called caprolactam, which contains 6 carbon atoms in its ring structure.
Chemical formula of caprolactam:
C₆H₁₁NO
When caprolactam undergoes ring-opening polymerization, it forms long chains of repeating units, each of which also contains 6 carbon atoms.
Repeat unit in Nylon 6:
css
复制
编辑
[-NH-(CH2)5-CO-]n
This structure consists of:
5 CH₂ (methylene) groups → 5 carbon atoms
1 carbon in the carboxylic acid group (CO) → 1 carbon atom
Total: 6 carbon atoms per monomeric unit
Thus, the “6” denotes the number of carbon atoms in the monomer used to create the polymer.
2.2 Linear vs. Condensation Polymerization
Nylon 6 is produced through ring-opening polymerization, a different mechanism from the condensation polymerization used in making other nylons like Nylon 6,6.
Nylon 6 → from one monomer (caprolactam)
Nylon 6,6 → from two monomers (hexamethylenediamine and adipic acid)
This difference affects properties such as crystallinity, melting point, and mechanical strength.
3. Nylon 6 vs. Nylon 6,6: A Structural Comparison
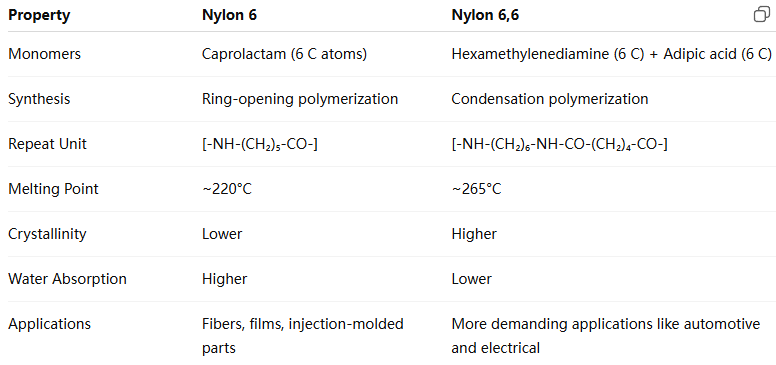
The naming conventions follow a systematic approach:
Single number (e.g., Nylon 6, Nylon 12): made from a single monomer with that many carbon atoms.
Two numbers (e.g., Nylon 6,6; Nylon 6,10): the first number refers to the number of carbon atoms in the diamine; the second refers to the number in the dicarboxylic acid.
4. Historical Context and Industrial Importance
4.1 Discovery and Motivation
Nylon 6 was first developed by Paul Schlack at IG Farben in Germany in 1938, as a response to DuPont’s invention of Nylon 6,6. Schlack wanted a polyamide that could be produced from a single monomer, allowing for easier synthesis and independence from DuPont's patented condensation process.
4.2 Applications
Today, Nylon 6 is widely used in:
Textiles and carpets: due to its resilience and dyeability
Engineering plastics: gears, bearings, housings
Films and packaging: for food and industrial use
Its abrasion resistance, impact strength, and cost-effectiveness make it indispensable across industries.
5. Conclusion: More Than Just a Number
The “6” in Nylon 6 is not arbitrary — it encodes crucial information about the chemical makeup, synthesis route, and physical properties of the polymer. It tells us that the polymer is made from a monomer with six carbon atoms and that it is a homopolymer formed via ring-opening polymerization of caprolactam.
Understanding this naming system helps chemists, engineers, and manufacturers choose the right nylon for the right application. As simple as it seems, the number “6” is a powerful key to unlocking the science behind one of the world’s most important synthetic materials.


 English
English 中文简体
中文简体 Español
Español عربى
عربى











